General introduction on phosphorus-based flame retardant
Types of phosphate flame retardant

Phosphorus-based (Phosphate esters) flame retardants can be categorized into two types based on their working methods: reactive and additive.
Reactive flame retardants achieve long-lasting flame retardancy by chemically bonding flame-retardant groups to other materials. On the other hand, additive phosphorus-based flame retardants are mixed with polymer materials and processed into composite materials with flame-retardant properties. Due to their convenience of use, additive flame retardants dominate the field of flame retardancy in polycarbonate (PC) applications.
Phosphorus-based flame retardants can be further classified into two major categories: inorganic and organic.
Inorganic phosphorus-based flame retardants mainly include red phosphorus, phosphates (such as ammonium dihydrogen phosphate, and diammonium phosphate), and ammonium polyphosphate. Organic phosphorus-based flame retardants encompass phosphate esters, phosphonate esters, DOPO derivatives, and phosphazene flame retardants.
Considering both flame retardant effectiveness and cost, organic phosphorus-based flame retardants are currently extensively employed in the realm of flame retardancy for organic materials.
Flame retardancy mechanisms
Organic phosphorus flame retardants exhibit flame retardancy through two primary mechanisms: vapor phase inhibition and solid phase char formation.
Solid phase char formation
1. During combustion, the flame retardant undergoes decomposition and catalyzes the dehydration of the polymer, leading to the formation of a carbonized layer.
2. The flame retardant interacts with oxygen and water during combustion, forming phosphoric acid, which further polymerizes into polyphosphoric acid and polyphosphoric esters. These compounds can adhere to the surface of the carbonized layer, assuming a glassy or gel-like state. This serves as a barrier, isolating oxygen and combustible materials.
Vapor phase inhibition
1. The decomposition of the flame retardant generates PO· radicals, while the polymer decomposition yields a small number of hydrogen radicals (H·) and hydroxyl radicals (HO·). The PO· radicals produced by the phosphorus-based flame retardant can capture H· and HO· radicals, forming HPO. This process inhibits or slows down the combustion chain reaction, thus enhancing the flame-retardant effects.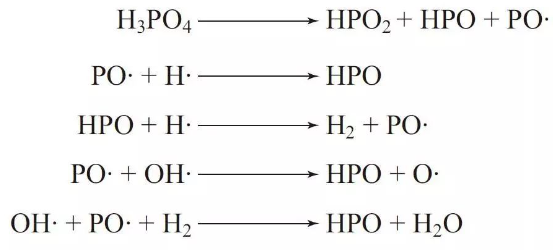
2. The flame retardant undergoes decomposition at high temperatures, producing non-flammable gases such as P, PO, and HPO. This reduces the concentration of combustible gases, thereby delaying the spread of the flame.
Derivatives of phosphorus flame retardant
Phosphate ester flame retardant
Phosphate ester flame retardants are mostly additive flame retardants that possess dual functions of flame retardancy and plasticization. The commonly used phosphorus-based flame retardants in the market include tris(2-chloroisopropyl)phosphate (TCPP), triphenyl phosphate (TPP), triethyl phosphate (TEP), resorcinol bis(diphenyl phosphate) (RDP), and bisphenol A bis(diphenyl phosphate) (BDP). These three flame retardants have slight differences in their flame retardant mechanisms. TPP tends to volatilize at high temperatures and can only exhibit flame retardancy through vapor phase inhibition. In contrast, RDP and BDP can exert their flame-retardant effects in both the vapor phase and the solid phase. However, one major drawback of phosphate ester flame retardants is their poor resistance to hydrolysis, which is a current limitation in their application in organic materials.
Phosphonate ester flame retardant
Current research on phosphonate ester flame retardants mainly focuses on nitrogen-doped phosphonate flame retardants and reactive phosphonate ester flame retardants. Phosphonate ester molecules exhibit good stability, excellent water resistance, and solvent resistance. They possess dual functions of flame retardancy and plasticization, making them suitable for flame retardant treatment of various polymer materials. Phosphonate ester flame retardants demonstrate good thermal stability, moderate pricing, effective flame retardant performance, minimal impact on material mechanical properties, and good resistance to hydrolysis. These characteristics make them promising for application in the field of flame retardancy.
DPPO flame retardant
The DPPO (9,10-Dihydro-9-oxa-10-phosphaphenanthrene) molecule exhibits excellent stability and good resistance to hydrolysis. It has many advantages, such as being halogen-free, non-toxic, and providing long-lasting flame retardancy. Compared to phosphate ester flame retardants, DPPO has a lesser impact on the thermal and mechanical properties of the resin matrix, making it a hot topic in flame retardant research for organic materials. It is generally believed that DPPO exhibits a gas-phase flame retardant mechanism. When exposed to high temperatures, DPPO releases phosphorus radicals and phenoxy radicals to quench the combustion chain reactions in the gas phase, thereby achieving flame retardancy. DPPO molecules with phosphorus-containing skeletons serve as good intermediates for the synthesis of flame retardants. By modifying the structure of DPPO, it is possible to synthesize novel flame retardant molecules with excellent flame retardant performance, offering broad prospects for applications in the field of flame retardancy.
Phosphazene flame retardant
Phosphazene molecules, rich in phosphorus and nitrogen elements, exhibit excellent synergistic flame-retardant effects, making them effective flame-retardant materials. Phosphazene molecules can generally be classified into cyclic phosphazenes and linear phosphazenes. The flame retardant mechanism of phosphazene flame retardants involves both gas phase and condensed phase flame retardancy. Cyclic phosphazene molecules, with a six-membered ring structure composed of alternating phosphorus and nitrogen atoms, have high thermal stability. Upon heating, they undergo thermal decomposition to produce polyphosphoric acid and polyphosphoric esters, which cover the surface of the char layer and act as solid-phase char formation flame retardants. Additionally, the combustion process generates phosphorus oxygen radicals and non-flammable gases (such as PO· and NH3), which can terminate radical chain reactions and dilute the concentration of combustible gases, thereby slowing down the further combustion of organic materials and demonstrating gas phase flame retardancy.
Hexachlorocyclotriphosphazene, with multiple reactive sites, can be chemically modified to prepare different types of cyclic phosphazene flame retardants. It serves as an ideal intermediate for the synthesis of flame retardants. Phosphazene flame retardants have advantages such as high flame retardant efficiency, low environmental pollution, and good dispersibility in PC materials, showing potential applications in the field of flame retardancy for PC. However, the production cost of these flame retardants is relatively high.
Inorganic phosphorus flame retardant
Currently, the application of inorganic phosphorus flame retardants in PC flame retardancy is limited. Inorganic phosphorus flame retardants have lower flame retardant efficiency and require the addition of 40% to 60% (wt) of flame retardants to achieve a V-0 flame retardancy rating in the UL-94 vertical burning test for PC products. However, increasing the additive amount significantly affects the mechanical properties of the material, leading to reduced strength, elastic modulus, compatibility, and stability. Therefore, it is necessary to improve the compatibility between the flame retardant and PC through modification of the additive to enhance the thermal stability of the product.
Partnering up with a global leading material manufacturer that supplies diversified phosphate esters chemicals.
